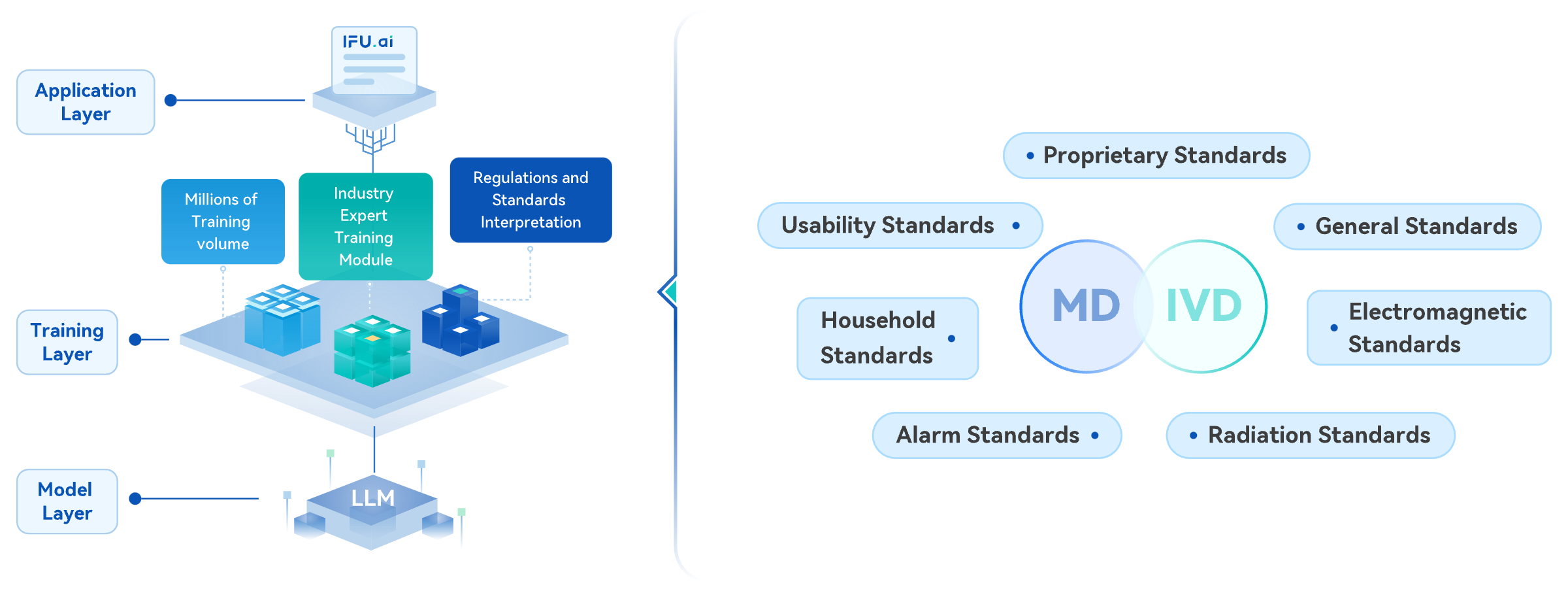
Pure Global GRIP Platform - IFU.ai
Pure Global's independently developed 'Automated Generation of Regulatory Document Series Services,' the flagship product, has trained data from millions of medical device instruction manuals and optimized and debugged large language models, taking the lead in introducing AIGC to the industry. This breakthrough overcomes knowledge barriers and greatly enhances work efficiency.

Writing Product Specifications is Never Easy
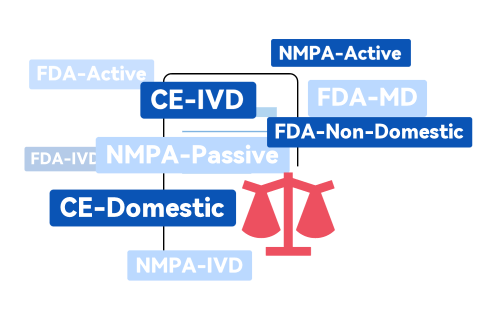
Typically,due to the complexity of the product specification system,it often requires constant communication between multiple departments,resulting in delays.
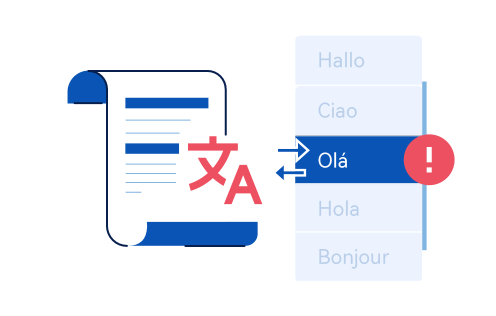
For example,in the EU,there are over 24 European Languages that need to be covered and translation expenses cannot be overlooked.
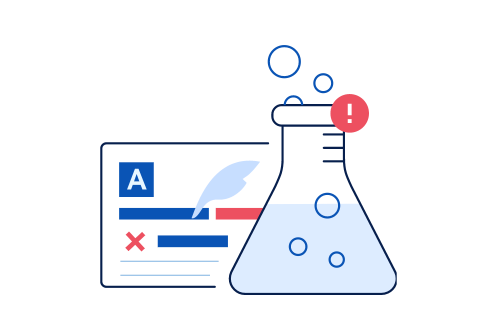
When conducting clinical trials,it can often be delayed due to the lack of information in the manual or language barrier.

Effective consumer communication relies heavily on product specifications as it is the sole means of the interaction between manufacturers and end consumers.
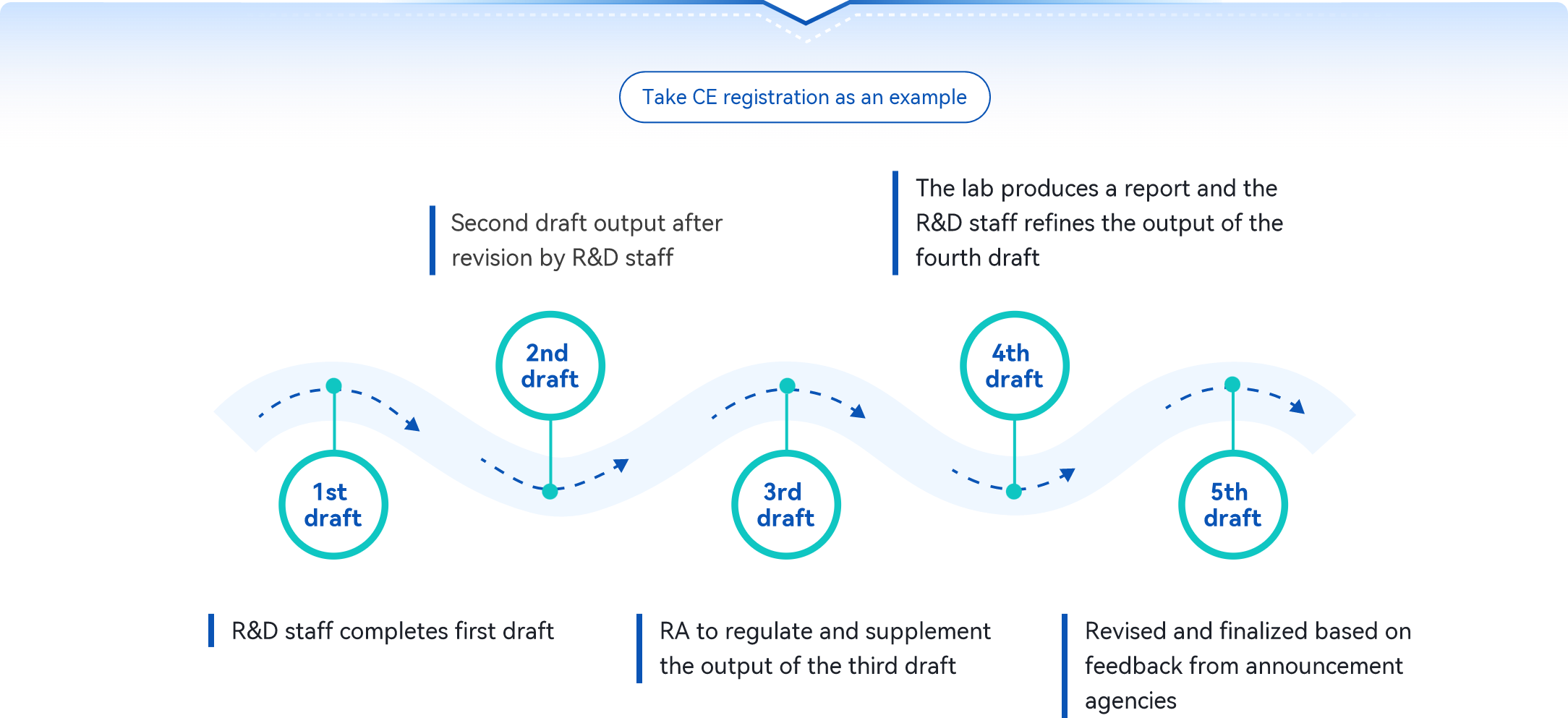
3 Major Function Update!
Full Review
Key Points
Terminology
Full Review
We'll Help You Spot and Fill in Any Missing Pieces
We'll Identify Missed Sections, Key Regulations, and Areas for Improvement in the Manual – All at the Click of a Button, Easing the Workload for Regulatory Affairs (RA) Staff.
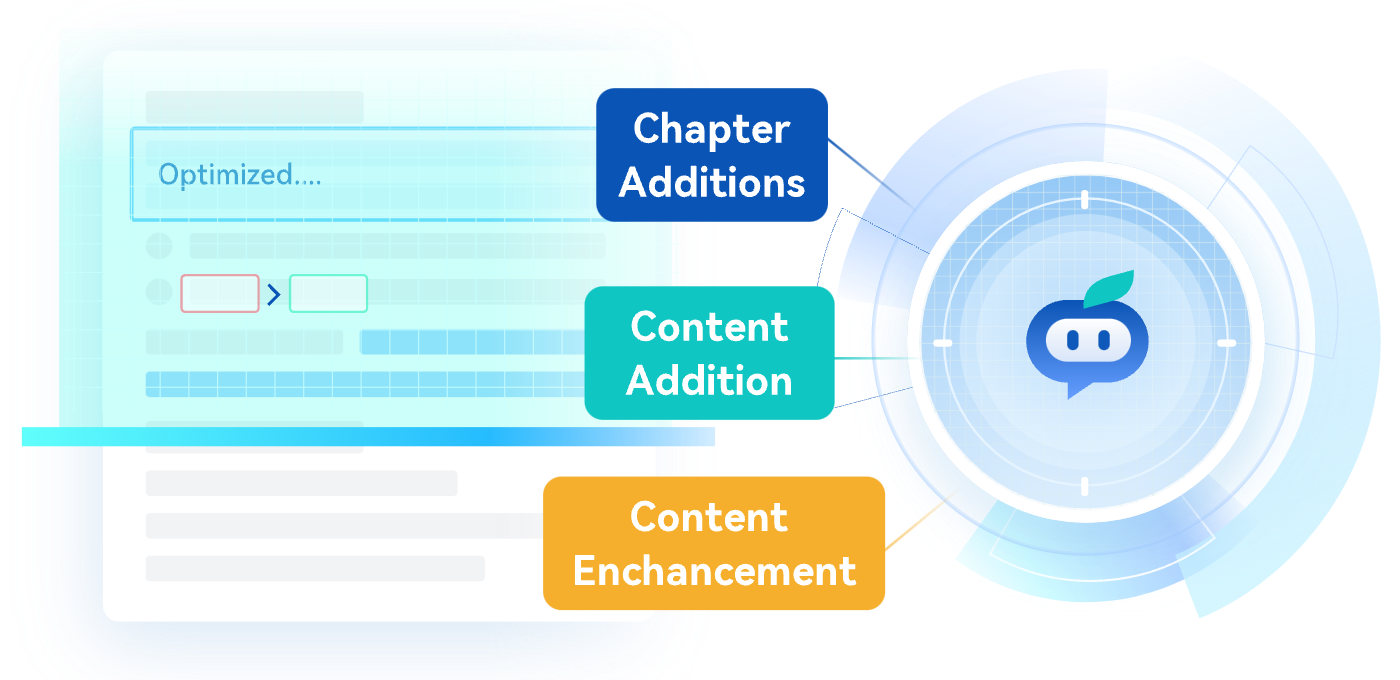
Service Introduction
 IFU Automatic Writing-Comprehensive
IFU Automatic Writing-Comprehensive
